Tendon mechanobiology
Tendons connect muscles to bones and are responsible for transferring mechanical loads and translating muscle contractions to joint movements. They can withstand high tensile loads and have complex load-dependent biomechanical properties. The Achilles tendon is the largest tendon in the body and the most commonly injured tendon. Treatments of ruptures is a complex medical problem that requires a controlled mechanical environment to restore the biomechanical strength and the load bearing capacity of the tissue. Understanding how loading can influence tendon homeostasis and healing is essential for maintaining and restoring the predominantly mechanical functions of tendons.
Click on the toggle bars to read more:
Our research is focused on the biomechanical and mechanobiological behavior of tendons and how these mechanical properties change due to loading and develop during healing, using both experimental and computational approaches. We have developed a 3D tendon computer model which enables us to investigate different tendon properties, e.g. tendon geometry, heterogeneous structure and viscoelastic mechanical behavior, of the tendon through finite element analysis. The computer model is validated with high-resolution imaging and mechanical data obtained from our experimental investigations of the rat Achilles tendon.
Current investigations focus on understanding mechano-regulation of the tendon healing process by modeling the evolution of tissue properties, e.g. tissue composition, tissue organization, and mechanical properties. A particular goal is to explore various mechanical and biological mechanisms underlying the heterogeneous distribution of tissues properties.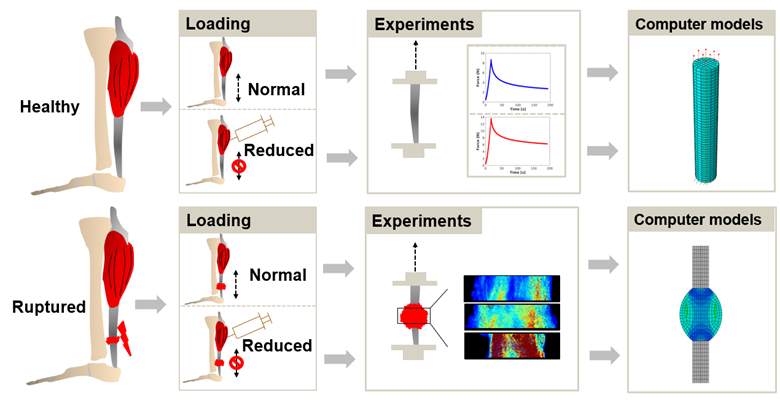
Figure: Experimental data from small animal studies on healthy and ruptured rat Achilles tendon are used for identifying tendon properties and validating our modeling predictions of the healing process. In particular, the effect of external mechanical loading on the evolution of tissue properties is investigated.
Researchers at LU: PI Hanna Isaksson, postdocs: Chiara Garavelli, Yeswanth Sai Pydi.
Funding: Knut and Alice Wallenberg KAW Foundation. The European Union’s Horizon 2020 program.
Recent publications (external links):
- Notermans, Khayyeri, Isaksson. Predicting the effect of unloading and cell infiltration on the spatio-temporal tissue distribution during Achilles tendon healing. Journal of Biomechanics, 110853, 2021 https://doi.org/10.1016/j.jbiomech.2021.110853
- Notermans, Hammerman, Eliasson, Isaksson: Tendon mechanobiology in small animal experiments during post-transection healing. European Cell and Materials 42:375-391 2021 DOI: 10.22203/eCM.v042a23
- Notermans, Tanska, Korhonen, Khayyeri, Isaksson: A numerical framework for mechano-regulated tendon repair – simulation of early healing of the Achilles tendon. PLOS Computational Biology 8;17(2):e1008636, 2021 https://doi.org/10.1371/journal.pcbi.1008636
Our aim is to unravel this relationship between mechanical stimuli and tendon structure. To do this, we need to understand how each structural level is affected and responds to mechanical stimuli, as well as the nature of load transfer between them.
In collaboration with Linköping University, we are using a small animal model, namely the rat. Intact and healing rat Achilles tendons are exposed to varies degree of loading (and unloading). To study the structure of the tendons at different hierarchical levels, we use high-resolution Synchrotron techniques combined with conventional methods, e.g. histology, microscopy and mechanical testing. At the tissue scale, tendons are imaged in 3D by phase contrast tomography, which enables us to study fiber orientation and local tissue damage. At the material scale, the structure is characterized in 2D by immunohistochemistry, scanning electron microscopy and Fourier-transform infrared spectroscopy. Additionally, small-angle X-ray scattering is used to study the collagen fibrils.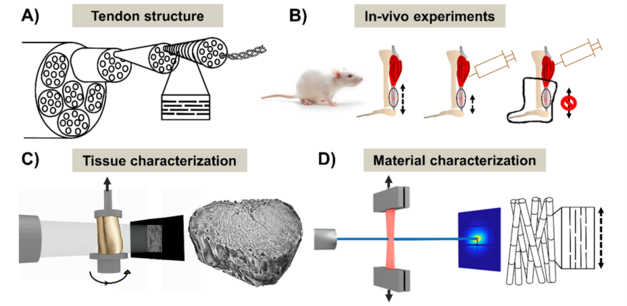
Figure: (A) Schematic representation of the Achilles tendon hierarchical structure. (B) Three levels of in vivo loading are studied. (C) At the tissue scale, tendons are imaged in 3D by phase contrast tomography. (D) At the material scale, the collagen is studied using e.g. X-ray scattering.
Researchers at LU: Researcher: Maria Pierantoni; PhD students Isabella Silva Barreto, Kunal Sharma; PI Hanna Isaksson
Collaborators: Dr Pernilla Eliasson, Sahlgrenska University Hospital, Gothenburg
Funding: Knut and Alice Wallenberg Foundation, European Research Council ERC, Kungliga Fysiografiska Sällskapet i Lund
Recent publications (external links):
Silva Barreto, Pierantoni, Hammerman, Törnquist, Le Cann, Diaz, Engqvist, Liebi, Eliasson, Isaksson, Nanoscale characterization of collagen structural responses to in situ loading in rat Achilles tendons, Matrix Biology 115: 32-47, 2023 https://doi.org/10.1016/j.matbio.2022.11.006
Pierantoni, Silva Barreto, Hammerman, Novak, Diaz, Engqvist, Eliasson, Isaksson, Multimodal and multiscale characterization reveals how tendon structure and mechanical response are altered by reduced loading, Acta Biomaterialia 168: 264-276, 2023 https://doi.org/10.1016/j.actbio.2023.07.021
Pierantoni, Silva Barreto, Hammerman, Verhoeven, Törnquist, Novak, Mokso, Eliasson, Isaksson: A quality optimization approach to image Achilles tendon microstructure by phase-contrast enhanced synchrotron micro-tomography. Scientific Reports 11, 17313, 2021 https://doi.org/10.1038/s41598-021-96589-w
