Bone Mechanics
Click on the toggle bars to read more:
Osteoporosis is defined as low bone mass, and results in a markedly increased risk of skeletal fractures. Development of new drugs to reduce bone loss or increase bone mass is promising. However, it requires that the individuals at risk can be accurately identified.
Our research aims at improving osteoporosis diagnosis and fracture risk assessment. This will be accomplished by combining DXA imaging, a pre-developed shape template, statistical shape modeling, and finite element analysis (FEA).
The project develops a method to estimate the 3D geometry of the hip bone based on a 2D image and a shape template. This method describes both the external geometry and the internal bone mineral density distribution. Thereafter, the shape template and the 2D image of the patient is used together with finite element analyses to assess the bone strength and the individuals' fracture risk.
Figure: Schematic of the proposed new method to predict fracture risk. From left to right, a 2D calibrated radiological image (DXA) is acquired in a clinical setting. The 2D image is then converted to a full 3D description of the hip using a shape template and the statistical shape modeling. 3D finite element models are built from the reconstructed shape, and the predicted femoral strength is used to determine the individual’s risk of fracture.
Researchers at LU: Assistant prof. Lorenzo Grassi. PI: Hanna Isaksson
Funding: Swedish Research Council, Mats Paulsson's foundation.
Recent publications (external links):
Grassi, Väänänen, Jehpsson, Ljunggren, Rosengren, Karlsson, Isaksson. 3d Finite Element Models Reconstructed from 2d Dxa Images Improve Hip Fracture Prediction Compared to Areal Bmd in Mros Sweden Cohort. Journal of Bone and Mineral Research 38:9, 1258-1267, 2023 https://doi.org/10.1002/jbmr.4878.
Grassi, Väänänen, Isaksson. Statistical shape and appearance models: development towards improved osteoporosis care. Current Osteoporosis Reports 19, 676–687, 2021 https://doi.org/10.1007/s11914-021-00711-w
Grassi, Fleps, Sahlstedt, Väänänen, Ferguson, Isaksson, Helgason: Validation of 3D finite element models from simulated DXA images for biofidelic simulations of sideways fall impact to the hip, Bone, 2021 doi.org/10.1016/j.bone.2020.115678
Osteoarthritis (OA) is one of the most common musculoskeletal disorders in adults, but several hip- related pathologies that increase the risk of OA appear already during growth. However, there are no well-defined thresholds to diagnose hip-related deformities during growth.
This proposal develops a novel approach to detect hip abnormalities during growth. The
hypothesis is that a combination of 1) accurate anatomical definition of the hip in 3D; 2) subject-specific predictions of the stress state of the hip cartilage, and 3) of the overall mechanical competence of the bones, can lead to a much-improved identification of hip developmental diseases and of the long-term risk for OA.
This assessment is clinically feasible thanks to a statistical shape and appearance model of the hip. The model works as an atlas of normal hip anatomy in 3D as a function of age and sex and allows to obtain 3D subject-specific models of pelvis, femur, and the articular cartilage from one 2D x-ray image.
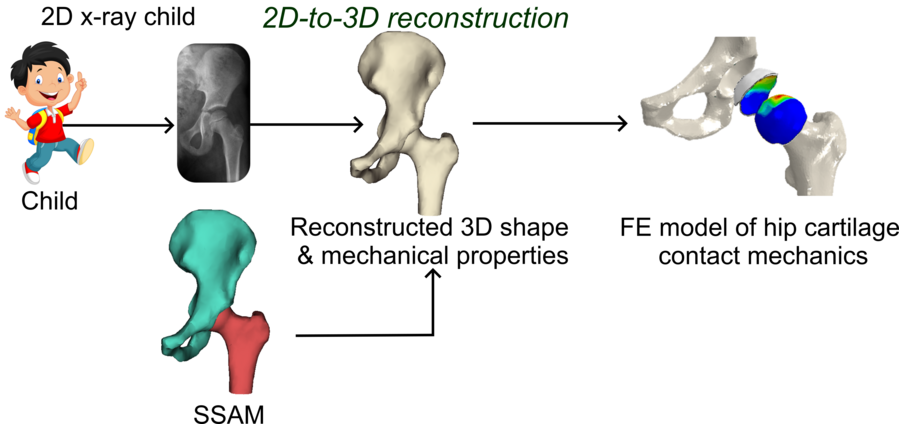
Figure: Schematic of the modelling pipeline to obtain 3D models of the full hip joint from 2D x-ray images of children.
Researchers at LU: PI: Lorenzo Grassi, PhD student: Shoaib Attar.
Funding: Swedish Research Council, Kockska’s foundation
Finite element (FE) models of the proximal femur (hip bone) can be used to accurately describe the mechanical behavior of the femur subjected to all kinds of loading scenarios. These models can be created based on clinical images and then be used to, for example, calculate the strength required to fracture a hip. To ensure that the models are as accurate as possible they should be validated against experimental measurements.
Our aim is to validate FE models for the accurate prediction of the mechanical behavior of the proximal femur. To be able to do this we have performed experiments where we loaded cadaveric femora until fracture and measured strains on the surface using high-speed cameras. We continue to develop FE models that we validate against these measurements.
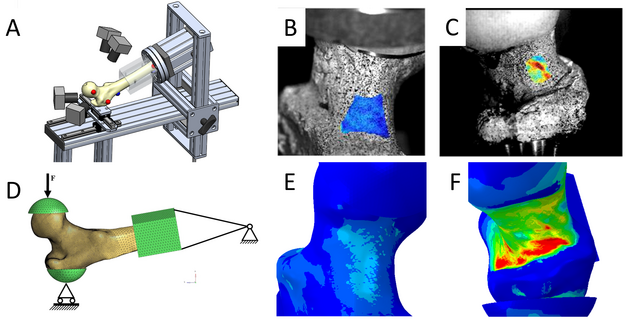
Figure: (A) Experimental setup used for mechanical testing, including 4 cameras used to record the deformation of the bone. (B,C) Pictures of a femur taken with high-speed cameras during mechanical testing (blue represents low deformation and red represents high deformation). (D) FE model of a proximal femur replicating the mechanical tests. (E,F) Deformations predicted by the FE model.
Researchers at LU: Assistant professor Lorenzo Grassi; Post-doc: Anna Gustafsson; PI: Hanna Isaksson
Funding: Swedish Research Council.
Recent publications (external links):
Kok, Odin, Rokkones, Grassi, Isaksson, The influence of foramina on femoral neck fractures and strains predicted with finite element analysis, Journal of the Mechanical Behavior of Biomedical Materials, 105364, 2022 https://doi.org/10.1016/j.jmbbm.2022.105364
- Kok, Grassi, Gustafsson, Isaksson: Femoral strength and strain in sideways fall: Validation of finite element models against bilateral strain measurements. Journal of Biomechanics, 9, 122, 110445, 2021 https://doi.org/10.1016/j.jbiomech.2021.110445
- Gustafsson, Tognini, Bengtsson, Gasser, Isaksson, Grassi: Subject-specific FE models of the human femur predict fracture path and bone strength under single-leg-stance loading, Journal of the Mechanical Behavior of Biomedical Materials, 2020 doi.org/10.1016/j.jmbbm.2020.104118
Bone is a composite material with a well-defined structure from the molecular building blocks up to the organ scale. Due to its unique hierarchical structure, bone can deform and dissipate energy at multiple length scales simultaneously and thereby protect the tissue from breaking. The most potent toughening mechanisms are found at the microscale, where the microstructure efficiently prevents crack growth by slowing down, stopping, and redirecting growing cracks. These protective mechanisms get impaired with age and diseases, such as osteoporosis, which makes the bone tissue more prone to fracture. However, the underlying mechanisms are not well understood.
Our aim is to understand how the fracture resistance of bone is affected by age or disease and to identify what features that are key for maintaining good mechanical integrity. To do this, we need to understand how bone, as a composite material, resists mechanical loading and how this is fundamentally altered with age and osteoporosis.
We are using computational fracture models to unravel the role of microstructure for crack growth in cortical bone. We have developed a framework for simulating crack propagation in heterogeneous bone tissue based on the extended finite element method (XFEM). The models include a criterion for interface damage to capture realistic crack patterns. These models are applicable at both the tissue- and microscales.
Our current effort is focused on exploring the use of phase field damage models to study damage mechanisms within the cortical microstructure. In phase field models, crack nucleation and propagation can be simulated with a minimum of material parameters. Unlike XFEM models, phase field methods straightforwardly extend to 3D, which allows us to study also the most efficient toughening mechanisms: those observed when a crack is propagation across the bone.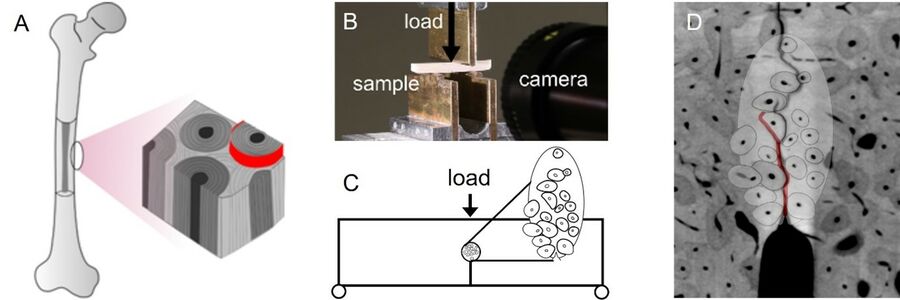
Figure: (A) Schematic illustration of the microstructure in cortical bone. (B) Experimental setup used for fracture-mechanical testing, including camera used to track the position of the crack tip. (C) Boundary conditions in computational model, including an embedded cell of resolved microstructure. (D) µCT-scan of fractured specimen overlaid with simulation result showing the simulated crack in red.
Researchers at LU: postdoc Jenny Carlsson; PI Anna Gustafsson
Funding: The Swedish Research Council, Royal Swedish Academy of Sciences, the Crafoord Foundation and the Foundation of Greta and Johan Kock, Royal Physiographic Society of Lund, Magnus Bergvall Foundation.
Recent publications (external links):
• Carlsson, Karlsson, Isaksson, Gustafsson. Phase-field simulation of crack growth in cortical bone microstructure: parameter identification and comparison against experiments. Biomech Model Mechanobiol, 2025. https://doi.org/10.1007/s10237-025-01929-8
• Gustafsson, Galteri, Barakat, Engqvist, Grassi, Cristofolini, Dejea, Isaksson. Characterization of damage mechanisms in cortical bone: Quantification of fracture resistance, critical strains, and crack tortuosity. Journal of the Mechanical Behavior of Biomedical Materials, 160: 106721, 2024 https://doi.org/10.1016/j.jmbbm.2024.106721
• Gustafsson, Isaksson. Phase field models of interface failure for bone application - evaluation of open-source implementations. Theoretical and Applied Fracture Mechanics. 121: 103432, 2022 https://doi.org/10.1016/j.tafmec.2022.103432
• Gustafsson, Wallin, Isaksson: The influence of microstructure on crack propagation in cortical bone at the mesoscale, Journal of Biomechanics, 2020 www.sciencedirect.com/science/article/pii/S0021929020304437
