Biomechanics Masters thesis suggestions
Interested?
If you are interested in any of the projects below, please contact Hanna Isaksson, hanna.isaksson@bme.lth.se
Development of phase-field fatigue model for simulating stress fractures in bone
Image analysis of cortical bone porosity – quantification and correlation to bone strength
Numerical models of the hip with individual loading conditions from motion lab
Open and reproducible patient-specific finite element (FE) models of the hip
Digital volume correlation (DVC) of phase contrast enhanced synchrotron X-ray tomographs of human menisci
Determining biochemical composition of articular cartilage with FTIR spectroscopy
Virtual histology of articular cartilage: Comparing synchrotrons and lab sources
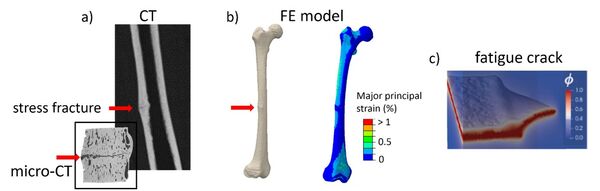
Development of phase-field fatigue model for simulating stress fractures in bone
Objective:
Develop a computational 3D finite element (FE) phase-field fatigue model for simulating stress fractures in bone. The aim is to simulate fatigue crack initiation and growth under daily loading conditions using a patient specific FE model.
Background:
Atypical femoral fractures (AFF) are stress fractures in the femoral shaft that mainly affect older osteoporotic patients on bisphosphonate (BP) therapy. This type of fatigue injury occurs due to repetitive daily loading and will lead to catastrophic failure when the crack has reached a critical size. Computational 3D FE models have shown that certain anatomical features in bone geometries (i.e., shaft bowing and neck-shaft angle) lead to a higher risk for AFF fractures, but no models exist that can simulate the fracture process itself.
Approach:
The candidate will develop phase-field fatigue FE models in 3D for simulating atypical femoral fractures. Available FE models of an AFF patient will be used as a starting point together with open-source code with the phase-field fatigue method implemented in Abaqus. Bone models of increasing complexity will be developed to simulate crack initiation and propagation under daily loading. The models will be used to explore what kind of fatigue mechanisms are prevalent, as well as estimate remaining load-carrying capacity and fatigue life for healthy and BP-treated bones that have been subjected to long-term repetitive loading.
Student background:
Knowledge in finite element modeling and solid mechanics. Knowledge of fracture mechanics is highly valued.
Important literature:
Kristensen, P. K., Golahmar, A., Martínez-Pañeda, E., & Niordson, C. F. (2023). Accelerated high-cycle phase field fatigue predictions. European Journal of Mechanics-A/Solids, 100, 104991.
Gustafsson, A., Schilcher, J., Grassi, L., Aspenberg, P., & Isaksson, H. (2016). Strains caused by daily loading might be responsible for delayed healing of an incomplete atypical femoral fracture. Bone, 88, 125-130.
For more info, please contact: Biomechanics group, Anna Gustafsson (anna.gustafsson@bme.lth.se) or Hanna Isaksson, hanna.isaksson@bme.lth.se
Image analysis of cortical bone porosity – quantification and correlation to bone strength
Objective:
The project will investigate the relationship between pore size in the femoral cortex and bone strength. The aim is to figure out whether an increased porosity can be linked to a decreased bone strength and, consequently, an increased fracture risk.
Background:
Ageing is associated with an increased fracture risk, the majority of which cannot be explained by changes in bone mass. One hypothesis is that the increased fracture risk is caused by an increased porosity in the cortex. Clinical studies have identified that patients who have experienced fragility fractures also tend to have larger and more numerous pores in the cortex. However, it is not known whether the size and/or distribution of pores can be directly linked to the mechanical strength of the bone.
Approach:
The candidate will perform image analysis on computed tomography image material obtained in a previous research project in which cadaveric human femurs were imaged before mechanical testing. The image data will be analysed to characterise porosity in the cortical shaft and femoral neck. Data from image analysis will be correlated to the experimentally measured failure strain in order to enhance the strength prediction in subject-specific finite element models by linking pore size to failure strength using relationships from fracture mechanics.

Student background:
Familiarity with image analysis (e.g. FMAN20) is required. Some knowledge about tissue biomechanics and finite element models (e.g. BMEN10) is also desirable.
Important literature:
Grassi et al. (2020) Elucidating failure mechanisms in human femurs during a fall to the side using bilateral digital image correlation. Journal of Biomechanics 106:109826
For more info, please contact: Biomechanics group, Anna Gustafsson (anna.gustafsson@bme.lth.se) or Lorenzo Grassi (lorenzo.grassi@bme.lth.se).
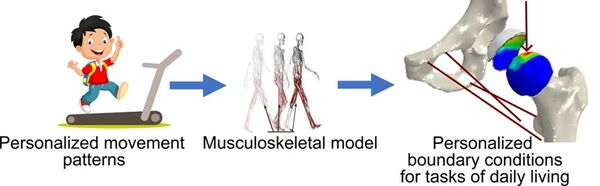
Numerical models of the hip with individual loading conditions from motion lab
Objective:
Develop a robust and automated pipeline to apply subject-specific boundary conditions on the hip joint based on recordings of motion from motion lab.
Approach:
Use the motion recordings from the MoRE-Lab (https://www.more-lab.lu.se) in Lund and develop a method to input their kinematic data into OpenSim software and run an inverse dynamics analysis to obtain muscle forces and joint reaction forces at the hip.
Background/Relevance/Application:
Numerical models of the hip can accurately predict the stress state in the cartilage for a given force. However, the different movement strategies adopted by different people result in very different loads acting on the hip joint. Adding relevant, subject-specific loads to a model of the hip is therefore crucial.
Student background:
Mathematics, basic knowledge in musculoskeletal modelling and/or data collection in motion capture labs.
Important literature:
Martelli et al. (2014) Strain energy in the femoral neck during exercise, Journal of Biomechanics
For more info, please contact: Biomechanics group, Lorenzo Grassi, lorenzo.grassi@bme.lth.se
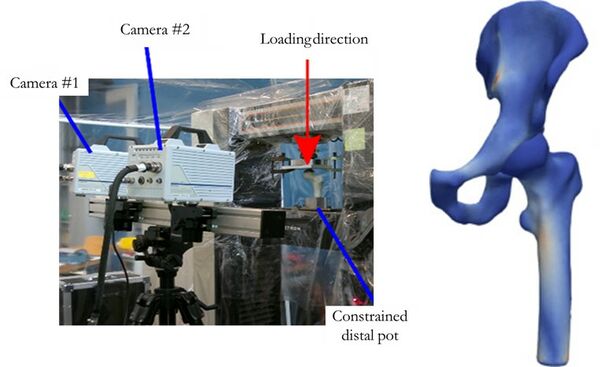
Open and reproducible patient-specific finite element (FE) models of the hip
Objective:
Develop an accurate and robust pipeline for subject-specific finite element modelling of human hips using open-source and free software.
Approach:
Starting from the current, validated, modelling pipeline in use in the Biomechanics group at Lund University, replace and adjust the different steps to use openly available tools for the model creation, solution, and post-processing.
Background/Relevance/Application:
The openness and reproducibility of research results has become a relevant point in the research community. Making pipelines open and reproducible can increase trustworthiness in the research community as well as allow more people to access those resources.
Student background:
Mathematics, finite element modelling and/or image processing, programming.
For more info, please contact: Biomechanics group, Lorenzo Grassi, lorenzo.grassi@bme.lth.se
Digital volume correlation (DVC) of phase contrast enhanced synchrotron X-ray tomographs of human menisci
Objective:
Characterize 3D strain and deformation in relation to the microstructural organization of fibers in human medial when dynamically compressed. Determine among the freely available algorithms for DVC which one is best performing to determine meniscus microstructural response to in situ loading. Compare mechanical properties of menisci form different donors.
Background:
Osteoarthritis (OA) is a "wear-and-tear" type of degenerative arthritis that mostly occurs in people over 50 years. Knee OA is associated with the degeneration of the meniscus. The meniscus, a crescent-shaped disc of fibrocartilage that is located between the surfaces of the femur and tibia in the medial and lateral compartments of the joint. It is composed of water and specifically hierarchically arranged collagen, which serves to distribute the load in the knee. Structural and mechanical changes are known to occur in the meniscus due to OA, however, the details of how the degeneration evolves are still unknown. Digital volume correlation (DVC) is a method to track translations of small sub-volumes between two subsequent 3D images. A matching is performed between subsequent loading-step volumes based on the similarity of the grayvalue patterns. DVC outputs are the internal displacements for multiple sub-volumes, based on which tensor strain fields can be computed and the evolution during loaded tracked. However, DVC was never performed on menisci imaged by dynamic phase contrast, so parameters will need specific optimization, and refined details of the expected outcome are unknown.
Approach:
The candidate will perform DVC of dynamic X-ray phase contrast imaging datasets of human meniscus. Focus is on identifying the best DVC algorithm and parameters to determine micro deformations and strain distribution in the tissue during loading.
Student background:
knowledge of 3D image analysis and programming.
For more info, please contact the Biomechanics group, Hanna Isaksson, hanna.isaksson@bme.lth.se

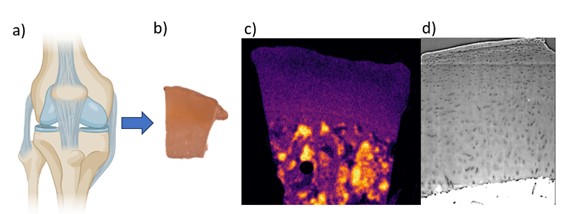
Determining biochemical composition of articular cartilage with FTIR spectroscopy
Objective:
Determine biochemical composition (such as collagen and proteoglycan content) of articular cartilage samples and compare to existing neutron and X-ray data.
Background/Relevance:
Our group is exploring novel methods of imaging cartilage to study osteoarthritis (OA) progression and have obtained promising images using both neutron and synchrotron X-ray tomography. Making full use of this data requires a comparison to a ground truth for the biochemical composition of the tissue using established spectroscopy techniques such as Fourier transform infrared (FTIR) spectroscopy.
Approach:
The candidate will prepare samples for FTIR measurements, including fixation and sectioning, and compare FTIR data to existing neutron and X-ray images.
Application:
Samples of human articular cartilage used in previous X-ray and neutron studies will be fixed and sectioned, before being measured with FTIR. Measured compositional data will be compared with gray values and other visible properties in neutron and X-ray images.
Student background:
Basic knowledge in image analysis as well as experience handling biological samples in the lab.
For more info, please contact: Biomechanics group, Hanna Isaksson, hanna.isaksson@bme.lth.se
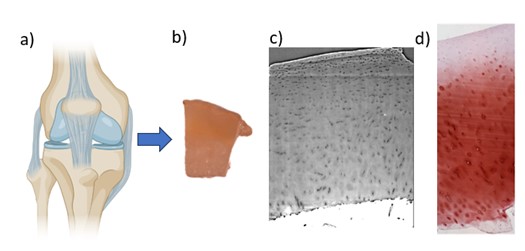
Virtual histology of articular cartilage: Comparing synchrotrons and lab sources
Objective:
Determine whether phase-contrast virtual histology of articular cartilage can be satisfactorily performed using a lab source.
Background/Relevance:
Histological staining and slicing is currently the gold standard for measuring the progression of osteoarthritis in articular cartilage biopsies. Phase-contrast-enhanced X-ray tomography provides the promise of making these measurements in a less destructive manner, as well as expanding the results to three dimensions, but has this far only been performed with synchrotron sources.
Approach:
The candidate will image articular cartilage samples previously imaged at a synchrotron using a lab source of X-rays, with the goal of obtaining results comparable in quality by using longer imaging times.
Application:
Samples of human articular cartilage will be imaged using a lab source and phase-contrast enhancement. The resulting images will be compared both to previous synchrotron images as well as traditional histological images in order to determine if comparative results can be achieved.
Student background:
Solid knowledge in image analysis as well as basic experience handling biological samples in the lab.
For more info, please contact: Biomechanics group, Hanna Isaksson, hanna.isaksson@bme.lth.se
